Électrodialyse bipolaire (EDBP)
Pour la récupération des courants de saumure afin de produire des produits acides ou caustiques
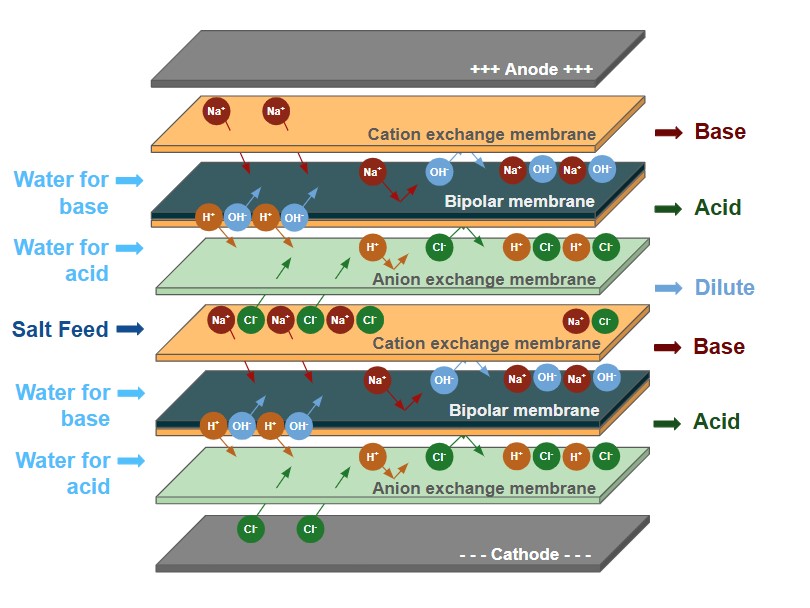
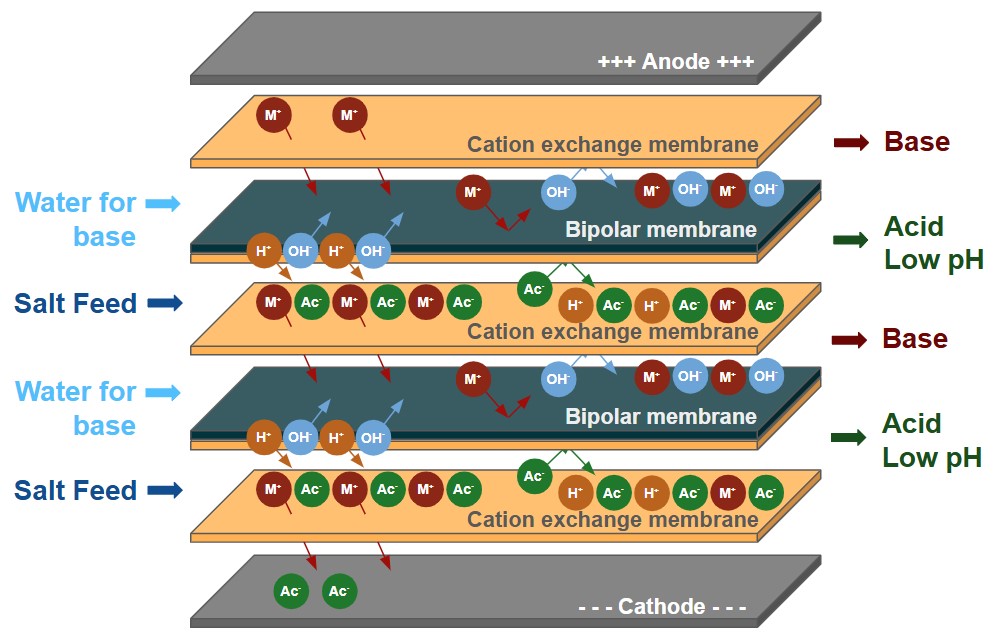
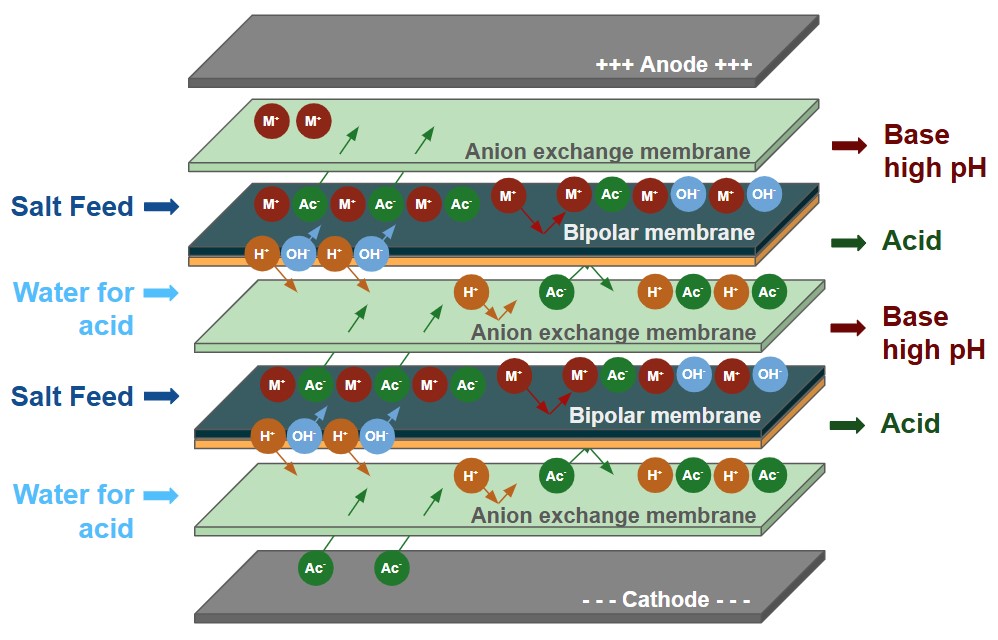
L'électrodialyse bipolaire utilise les bases de l'électrodialyse en y ajoutant une membrane bipolaire qui fractionne l'eau en H+ et en OH- à l'intérieur de la colonne d'électrodialyse. This allows the removed salts to be converted in their acid and/or base products.
Le BPED de Veolia peut être utilisé dans de nombreuses applications, telles que la production d'acides organiques, l'ajustement du pH dans les boissons ou l'utilisation de saumures industrielles pour générer des sous-produits acides et caustiques à valeur ajoutée.